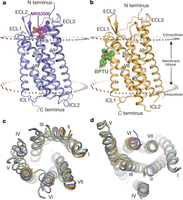
Structures of the P2Y1R–MRS2500 and P2Y1R–BPTU complexes.
Beili Wu 及同事报告了人P2Y1受体(一种 “G-蛋白耦合受体”,简称GPCR)的X-射线晶体结构。同P2Y12受体一样,这种膜蛋白也调控血小板激活和血栓形成。这两种GPCR都是新型抗血栓药物的研发工作的重要目标。将这一结构与一个以前发表的P2Y12受体结构所做对比表明,这两种GPCR的正构配体结合点是很不相同的:P2Y1受体的结合点要比P2Y12受体的结合点浅得多。作者还获得了该蛋白在核苷酸拮抗剂MRS2500和非核苷酸拮抗剂BPTU存在时的结构。MRS2500在正构点结合,但BPTU则与GPCR/类脂双层界面上一个不寻常的袋相结合。
原文标题:Two disparate ligand-binding sites in the human P2Y1 receptor
原文摘要:In response to adenosine 5′-diphosphate, the P2Y1 receptor (P2Y1R) facilitates platelet aggregation, and thus serves as an important antithrombotic drug target. Here we report the crystal structures of the human P2Y1R in complex with a nucleotide antagonist MRS2500 at 2.7 Å resolution, and with a non-nucleotide antagonist BPTU at 2.2 Å resolution. The structures reveal two distinct ligand-binding sites, providing atomic details of P2Y1R's unique ligand-binding modes. MRS2500 recognizes a binding site within the seven transmembrane bundle of P2Y1R, which is different in shape and location from the nucleotide binding site in the previously determined structure of P2Y12R, representative of another P2YR subfamily. BPTU binds to an allosteric pocket on the external receptor interface with the lipid bilayer, making it the first structurally characterized selective G-protein-coupled receptor (GPCR) ligand located entirely outside of the helical bundle. These high-resolution insights into P2Y1R should enable discovery of new orthosteric and allosteric antithrombotic drugs with reduced adverse effects.

